Hepatocellular carcinoma (HCC) is one of the most lethal malignancies worldwide, marked by late diagnoses, high recurrence rates, and limited treatment options. Preclinical models of liver cancer are essential for bridging the gap between laboratory discoveries and clinical therapies. Our contract research organization offers a comprehensive panel of HCC models – including murine allografts (syngeneic mouse tumor models) and human xenografts – to facilitate drug efficacy testing, tumor biology studies, immuno-oncology research, and precision medicine approaches. By leveraging both immunocompetent mouse models and human tumor grafts, researchers can investigate novel therapeutics in contexts that mirror the complex biology of liver cancer. Each model comes with distinct biological background and methodological advantages, enabling tailored studies on HCC pathophysiology, immune interactions, and targeted treatment responses.
H22 Allograft Model (Murine HCC, BALB/c)
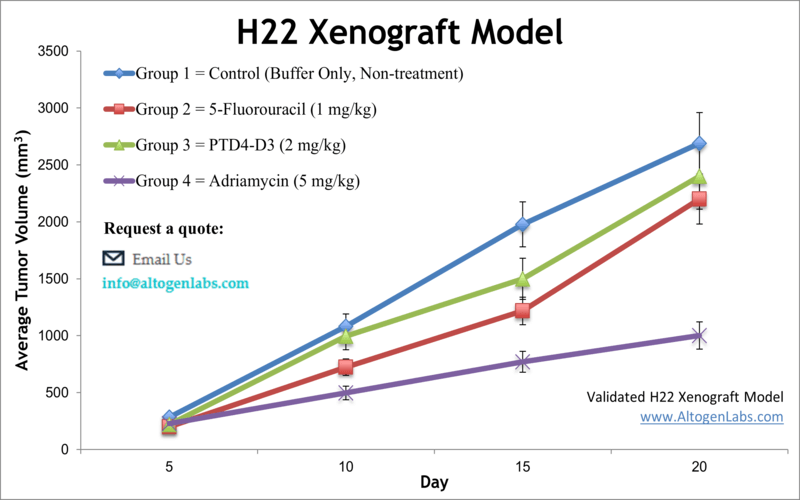
The H22 allograft is a classic immunocompetent liver cancer model derived from a murine hepatoma. Originally developed in BALB/c mice, H22 tumor cells can be engrafted into syngeneic mice (often BALB/c) where they robustly form tumors. This model is prized for its high tumor take rate and aggressiveness – H22 tumors grow invasively and can even exhibit metastatic behavior in vivo. Because the host mice have an intact immune system, H22 allografts are ideal for immuno-oncology studies: researchers can observe how the immune system interacts with the tumor and evaluate immunotherapies such as cancer vaccines or checkpoint inhibitors. For example, co-inhibition of the PD-1 and CTLA-4 checkpoints in H22 tumor-bearing mice was shown to significantly suppress tumor growth and enhance anti-tumor immune activity. Investigators also use H22 models to explore drug efficacy of new chemotherapy agents and targeted therapies. In one study, a novel sorafenib derivative (HLC-080) was tested in the H22 model and demonstrated potent tumor growth inhibition, underscoring H22’s utility for screening anti-cancer compounds. Overall, the H22 allograft provides a physiologically relevant platform for HCC research, allowing simultaneous assessment of tumor-intrinsic effects and immune responses in a single model.
Learn more about H22 Allograft Model
Hepa 1-6 Allograft Model (Murine HCC, C57BL/6)
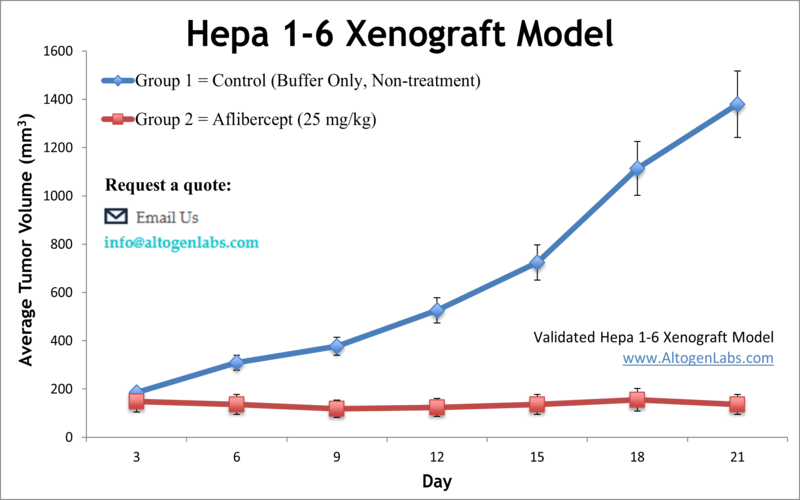
The Hepa 1-6 allograft is another syngeneic mouse liver cancer model, originating from a spontaneous hepatoma in a C57BL/6 (B6) mouse. As a tumor line on a B6 background, Hepa 1-6 can be transplanted into immunocompetent C57BL/6 mice without rejection, forming malignant liver tumors. A key advantage of the Hepa 1-6 model is its capacity to faithfully recapitulate tumor–immune system interactions in vivo. Researchers routinely employ Hepa 1-6 allografts to study how the immune system influences HCC progression and to test immunotherapies (such as checkpoint inhibitors, cytokine modulators, or cell-based therapies) in a relevant setting. For instance, this model has been used to evaluate novel immunotherapeutic compounds that mobilize T cells against HCC, leveraging the fully functional immune milieu. Beyond immunology, the Hepa 1-6 model is widely used for drug efficacy testing and investigations into HCC biology. The tumors arising from Hepa 1-6 cells carry molecular features of HCC and respond to standard anti-cancer agents, making this model suitable for assessing new chemotherapy drugs, targeted inhibitors, or combination treatments. Because it is an in vivo system, Hepa 1-6 also enables studies of tumor growth kinetics, metastasis potential, and drug resistance mechanisms under conditions that mirror a live host environment. In summary, the Hepa 1-6 syngeneic model offers a robust and immunologically relevant platform for preclinical HCC research – one that is particularly valuable for immuno-oncology and for therapies that require an intact immune context.
(Applications: Hepa 1-6 tumors are frequently used in evaluating immune checkpoint inhibitors, cancer vaccines, and other immunotherapies for HCC, as well as in conventional anti-cancer drug trials. Its B6 mouse compatibility also makes it a standard model for genetic and transgenic studies in immunocompetent hosts.)
Learn More about Hepa 1-6 Allograft Syngeneic Model
Hep3B Xenograft Model (Human HBV-associated HCC)
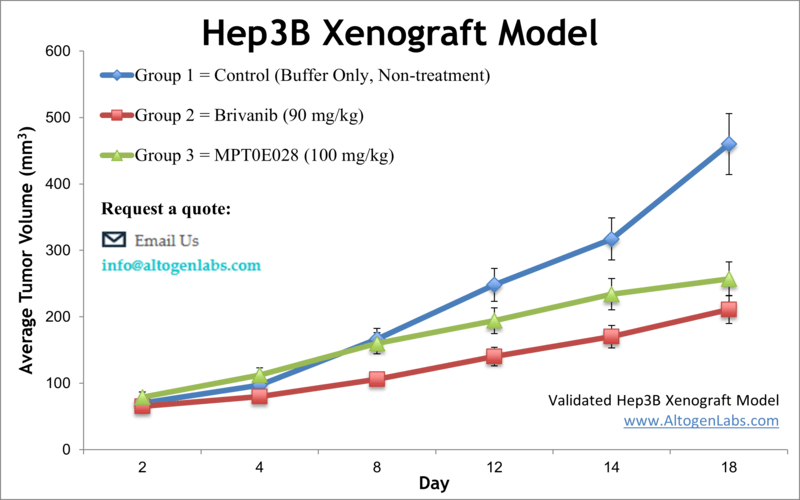
The Hep3B xenograft model uses a human HCC cell line (Hep3B) implanted in immunocompromised mice (typically nude or SCID mice). Hep3B cells were originally isolated from an 8-year-old patient with HCC and notably harbor integrated hepatitis B virus DNA. Biologically, this cell line represents an HBV-associated hepatocellular carcinoma, and it lacks a functional p53 gene (a characteristic that can influence its tumor behavior). In vivo, Hep3B readily forms tumors in nude mice, providing a reliable platform for drug efficacy testing against human liver cancer. Researchers have extensively used the Hep3B xenograft to evaluate targeted therapies, chemotherapeutic agents, and experimental treatments. For example, treatment with the mTOR inhibitor everolimus significantly inhibited Hep3B tumor growth in mice, and combinations like everolimus plus a microtubule inhibitor showed even greater effect, highlighting how this model can demonstrate therapeutic synergy. Similarly, anti-angiogenic agents (e.g. tyrosine kinase inhibitors like brivanib) have been tested in Hep3B xenografts, revealing reductions in tumor vascularization and proliferation. These studies underscore Hep3B’s relevance for HCC pathophysiology and drug response research – it captures aspects of tumor biology linked to viral etiology and allows testing of novel interventions in a controlled setting. While Hep3B xenografts lack an adaptive immune system (due to the use of immunodeficient mice), they remain invaluable for investigating tumor-intrinsic mechanisms, such as signaling pathways, apoptosis, and metabolism, under treatment. This model has even been utilized in imaging and molecular studies (for instance, PET imaging with labeled probes) to improve HCC detection and to monitor therapeutic effects in vivo. In summary, the Hep3B xenograft is a versatile human HCC model that excels in drug efficacy trials and mechanistic studies, particularly relevant for HCC cases with viral background or p53 pathway disruptions.
Learn More about Hep3B Xenograft Model
HepG2 Xenograft Model (Human Well-Differentiated HCC)
The HepG2 xenograft model is based on the HepG2 human liver cancer cell line, which originates from a well-differentiated hepatocellular carcinoma (in a 15-year-old patient). HepG2 cells are renowned for retaining many hepatocyte-like functions – they express various liver enzymes and proteins (such as albumin, insulin, and IGF-II) and can undergo hepatic differentiation. These traits make HepG2 an exceptional model for studying liver-specific metabolism and drug biotransformation in the context of cancer. In xenograft studies, HepG2 tumors are typically grown in immunodeficient mice (often with the aid of an extracellular matrix gel or orthotopic implantation, since HepG2 is less aggressive). Despite being a relatively slow-growing, differentiated tumor, the HepG2 model has shown value in preclinical drug testing, especially for therapies targeting metabolic pathways or proliferation signals. For instance, one study demonstrated that combining sorafenib (a multikinase inhibitor) with an SK2 sphingosine kinase inhibitor significantly slowed HepG2 tumor growth, suggesting a synergistic therapeutic strategy. Such findings illustrate how the HepG2 xenograft can be used to evaluate combination therapies and uncover interactions between pathways like the MAPK and sphingolipid signaling networks. This model has also been employed to explore HCC pathophysiology under various conditions – for example, it has helped validate mechanisms linking metabolic factors (like high glucose levels) to cancer progression via Wnt/β-catenin signaling. Additionally, because HepG2 cells share features with pediatric liver tumors (hepatoblastoma), orthotopic HepG2 models have been used to bridge research between HCC and childhood liver cancers. In a precision medicine context, the HepG2 xenograft’s distinct molecular profile allows investigators to test therapies on a less aggressive, differentiated tumor subtype, complementing the more aggressive models. Overall, the HepG2 model is a widely used tool in liver cancer research, valued for its hepatic functionality and utility in testing systemic treatments, anti-angiogenic agents (like bevacizumab), and novel drug combinations.
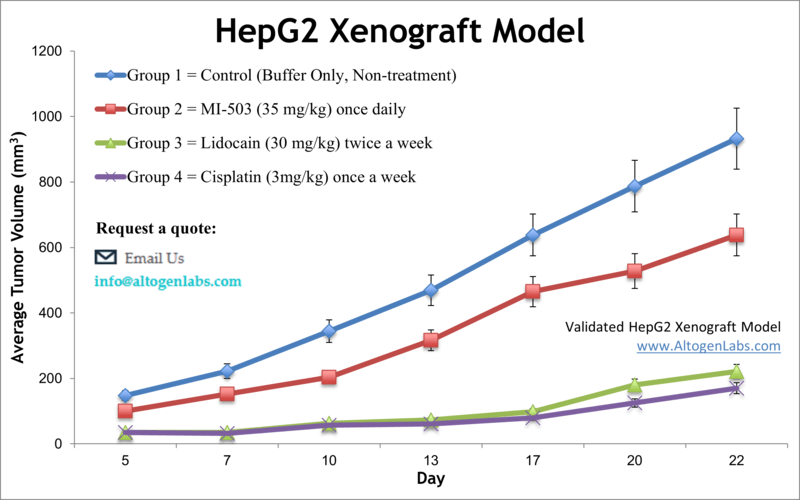
Learn more about Hep G2 Xenograft Model
Huh7 Xenograft Model (Human HCC with Viral Research Utility)
The Huh7 xenograft model uses the human Huh-7 cell line, a tumor line established from a Japanese male patient with HCC. Huh7 cells are especially notable for their unique virological properties: they can produce infectious hepatitis C virus in culture, a rare capability among cell linesaltogenlabs.com. This has made Huh7 a cornerstone for studying HCV biology and antiviral drugs. In cancer research, Huh7 xenografts in immunodeficient mice provide a platform to investigate HCC tumors that often have an intermediate differentiation status and a background of possible viral association (HCV). Scientists utilize Huh7 models to dissect molecular mechanisms of liver carcinogenesis, identify therapeutic targets, and evaluate new treatments. For example, using the Huh7 xenograft, researchers showed that an Aurora kinase inhibitor (VE-465) potently suppresses tumor growth by inducing apoptosis, paving the way for clinical exploration of Aurora kinase as a target in HCC. Huh7 tumors have also been used to test gene therapy approaches: one study delivered an artificial microRNA via oncolytic adenovirus to knock down the survival protein survivin, resulting in increased tumor cell apoptosis and growth arrest. These findings highlight Huh7’s role in evaluating innovative therapies ranging from small-molecule kinase inhibitors to viral vector-based treatments. Moreover, Huh7 carries certain mutations (for instance, in the beta-catenin gene) and has a high iron-storage capacity, features that make it a useful model for specific HCC subtypes (such as those involving Wnt signaling or dysregulated iron metabolism). In the context of drug testing, the Huh7 xenograft is used for assessing targeted agents like FGFR4 inhibitors or experimental compounds (e.g. plant-derived molecules), often showing clear tumor responses in vivo. While the model itself is immunocompromised, it can be combined with humanized mouse techniques or immune-modulating therapies (like oncolytic viruses) to glean insights into immunotherapeutic effects. Overall, the Huh7 xenograft stands out as a technically versatile HCC model – one that bridges virology and oncology – allowing researchers to study both conventional anti-tumor drugs and virus-targeted interventions in liver cancer.
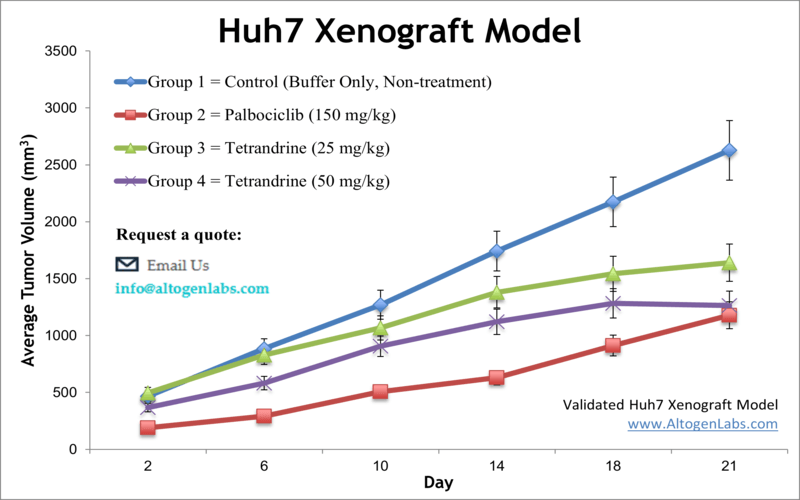
Learn More about HUH-7 Xenograft Model
SK-HEP-1 Xenograft Model (Human Liver Cancer with Mesenchymal Phenotype)
The SK-HEP-1 xenograft model is derived from the SK-HEP-1 cell line, an atypical human liver cancer cell line originally isolated from the ascites of a patient with liver adenocarcinoma. SK-HEP-1 is unique among HCC models because it exhibits a mesenchymal, highly invasive phenotype rather than the typical hepatocyte-like characteristics. In fact, gene expression analyses suggest SK-HEP-1 cells resemble liver sinusoidal endothelial or progenitor cells, lacking liver-specific markers but showing robust migration and tubular network formation. This unusual biology translates into the xenograft setting: SK-HEP-1 tumors grow aggressively in immunodeficient mice and have a pronounced capacity for invasion and metastasis. Researchers value the SK-HEP-1 model particularly for metastasis studies. It has been used to examine how tumors spread and to test anti-metastatic therapies, something not easily assessed in more static tumor models. In one notable study, SK-HEP-1 xenografts enabled simultaneous observation of primary tumor growth and spontaneous metastasis; the study identified a RNA-binding protein (Zcchc11) that drives both tumor expansion and dissemination, suggesting new therapeutic strategies to curb metastasis. In terms of molecular profile, SK-HEP-1 carries mutations in BRAF and CDKN2A, which is unusual for HCC and provides a model to test targeted inhibitors that might benefit rare subsets of liver cancer patients. This cell line’s intrinsic resistance to some conventional drugs (due to its dedifferentiated nature) also makes it a valuable model for studying treatment-resistant HCC. Indeed, SK-HEP-1 xenografts have been employed to evaluate next-line therapies: for example, experiments with the MET/VEGFR2 inhibitor cabozantinib showed it could overcome sorafenib resistance in this model, as evidenced by reduced angiogenesis, suppressed tumor growth, and increased apoptosis in SK-HEP-1 tumors. Additionally, other studies using SK-HEP-1 have implicated proteins like gp96 (an ER chaperone) in promoting tumor invasiveness, with silencing of such targets leading to diminished tumor growth and metastasis. These findings demonstrate how SK-HEP-1 serves as a platform for both drug efficacy and mechanistic discovery, especially concerning aggressive and metastatic disease. In summary, the SK-HEP-1 xenograft model offers a distinct angle in HCC research: it replicates a more mesenchymal, hard-to-treat form of liver cancer, thereby complementing other models and aiding the development of therapies for advanced HCC and metastasis-prone tumors.
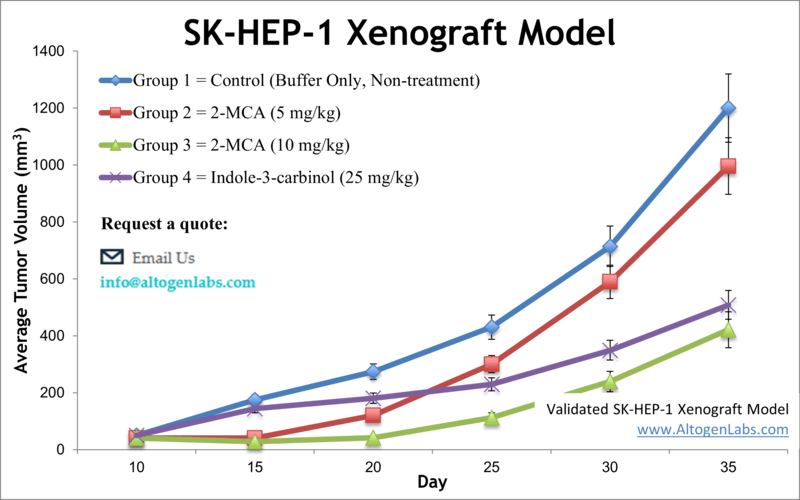
Learn More about SK-HEP-1 Xenograft Model
SNU-398 Xenograft Model (Human HCC – Advanced Stage and Combination Therapy Model)
The SNU-398 xenograft model utilizes the SNU-398 human liver cancer cell line, which was established from an advanced HCC in a Korean patient. This cell line is representative of a more aggressive, treatment-experienced tumor (the patient had undergone arterial chemoembolization and chemotherapy before the tumor was obtained). In immunocompromised mice, SNU-398 cells form tumors that have been instrumental in testing novel therapies and drug combinations for late-stage HCC. Researchers often choose the SNU-398 model to evaluate multi-targeted treatment strategies. A hallmark study combined several pathway inhibitors on SNU-398 tumors – pairing sorafenib (standard of care TKI) with agents like a MEK inhibitor (AZD6244) or an mTOR inhibitor (temsirolimus). The combinations yielded marked tumor growth inhibition and even regression, outperforming single agents. These results underscored the potential of combination regimens to tackle HCC’s complex signaling network, and SNU-398 xenografts provided the proof-of-concept. Another key application of SNU-398 is in studying targeted therapies for advanced HCC: for instance, the model was used to demonstrate how lenvatinib, a multi-kinase inhibitor recently approved for HCC, suppresses tumor progression. In vivo, lenvatinib treatment of SNU-398 tumors significantly blocked fibroblast growth factor receptor (FGFR) signaling and angiogenesis (shown by reduced microvessel density and downstream ERK phosphorylation). These findings align with clinical observations and help elucidate lenvatinib’s mechanism in a controlled setting. From a precision medicine perspective, SNU-398 has been linked to a specific molecular subclass of HCC – one characterized by active FGF/FGFR pathways and markers like E-cadherin and AFP secretion. Studies have revealed that this subclass (to which SNU-398 belongs) is particularly sensitive to FGFR inhibitors (such as BGJ398 and AZD4547), which potently curb SNU-398 tumor proliferation via MAPK pathway blockade. This exemplifies how cell line xenografts can guide targeted therapy for defined genetic subgroups of HCC, thus contributing to precision oncology. In addition to targeted drugs, SNU-398 xenografts are used for exploring resistance mechanisms and second-line treatments. Because HCC patients often receive multiple lines of therapy, the SNU-398 model’s history and behavior make it suitable for testing how tumors might respond to new agents after standard treatments. In summary, the SNU-398 xenograft is a powerful model for late-stage HCC, enabling the evaluation of combination therapies, the dissection of multi-kinase drug effects, and the tailoring of treatments to tumor subtypes in preclinical research.
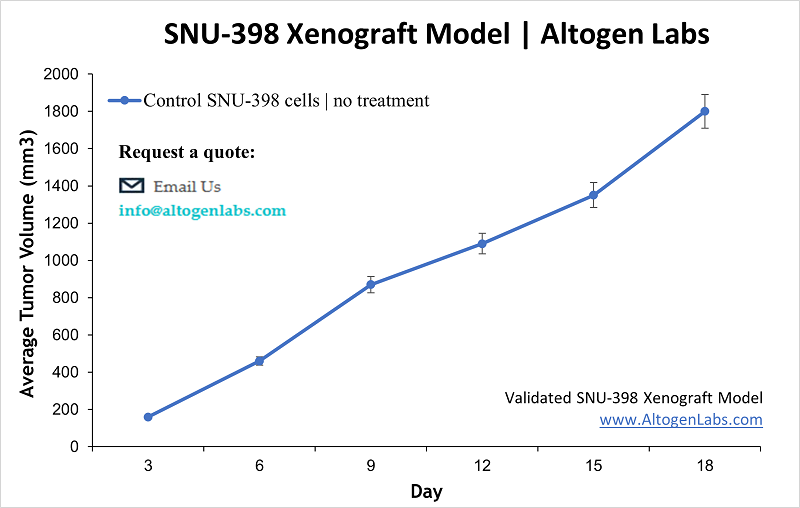
Learn more about SNU-398 Xenograft Model
SMMC-7721 Xenograft Model (Human HCC – Broad Utility and Novel Therapies)
The SMMC-7721 xenograft model is based on the SMMC-7721 human liver cancer cell line, established from a Chinese patient’s HCC tumor. SMMC-7721 is a widely used HCC model in Asia and globally, known for its utility in studying fundamental tumor biology as well as testing a wide array of therapeutics. The cell line expresses key growth factor receptors (such as EGFR and VEGFR) that are frequently involved in HCC progression, making it a relevant system for evaluating drugs targeting those pathways. In immunodeficient mice, SMMC-7721 forms reliable subcutaneous tumors, providing an economical and reproducible xenograft model for liver cancer. Researchers have leveraged SMMC-7721 xenografts in numerous innovative studies. For example, an anti-cancer compound derived from Croton plants (a taspine derivative, Tas-13D) was shown to significantly inhibit SMMC-7721 tumor growth in vivo, demonstrating the model’s role in screening novel drug candidates. Similarly, SMMC-7721 models have facilitated the discovery of new molecular targets: a landmark study identified a long non-coding RNA (lncRNA-ATB) activated by TGF-β in HCC using SMMC-7721 tumors, revealing that this lncRNA drives epithelial–mesenchymal transition, invasion, and IL-11/STAT3 signaling – thus pointing to lncRNA-ATB as a potential anti-metastatic target. The flexibility of SMMC-7721 is evident in the breadth of therapies tested. Investigators have used this model to evaluate everything from microbial metabolites to herbal extracts for anti-tumor efficacy. For instance, the bacterial metabolite borrelidin was found (in SMMC-7721 xenografts) to induce a MAPK-mediated tumor growth suppression and cell cycle arrest. In another study, a traditional Chinese medicine formulation (Dahuang Zhechong pill) was shown to overcome doxorubicin resistance in SMMC-7721 tumors by upregulating pro-apoptotic proteins and altering cancer cell metabolism. These examples highlight how the SMMC-7721 model supports research into drug resistance mechanisms and non-standard therapies. Importantly, this model is routinely used for testing low-toxicity or precision therapeutics and for combination regimens that include standard HCC drugs. Researchers have combined experimental agents (like the peptide LfcinB-P13 or the dietary compound 6-shogaol) with the standard-of-care sorafenib in SMMC-7721 xenografts, and even added targeted antibodies (such as an anti-EGFRvIII monoclonal antibody) to the mix – evaluating synergistic effects on tumor suppression. Such studies in the SMMC-7721 model have yielded insights into optimizing HCC therapy by enhancing efficacy while managing toxicity. In summary, the SMMC-7721 xenograft is a highly versatile preclinical model that lends itself to a variety of research applications: from decoding HCC molecular drivers to screening cutting-edge treatments and combination strategies that advance the goals of precision medicine in liver cancer.
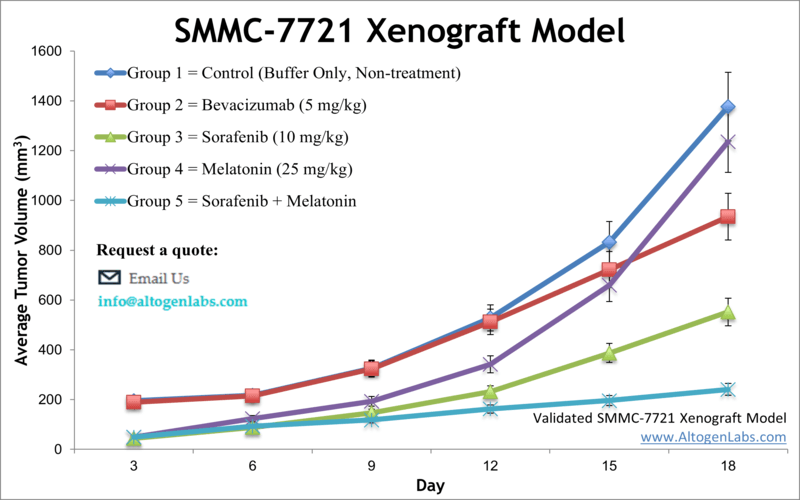
Conclusion: Our suite of HCC models – spanning murine allografts like H22 and Hepa 1-6, and human xenografts such as Hep3B, HepG2, Huh7, SK-HEP-1, SNU-398, and SMMC-7721 – provides an expansive toolkit for liver cancer research. Each model offers unique strengths, whether it’s engaging an intact immune system for immunotherapy studies or replicating specific molecular subtypes of HCC for targeted drug testing. By selecting the appropriate model(s), scientists can tailor preclinical studies to their therapeutic goals, be it testing the efficacy of a new drug, unraveling the mechanisms of HCC progression, or identifying the best treatment approach for a particular genetic profile of tumor. Ultimately, these models help accelerate the development of effective therapies for liver cancer by bridging laboratory findings with clinically relevant in vivo evidence, all within a technically supported, GLP-compliant CRO environment dedicated to advancing hepatocellular carcinoma research.